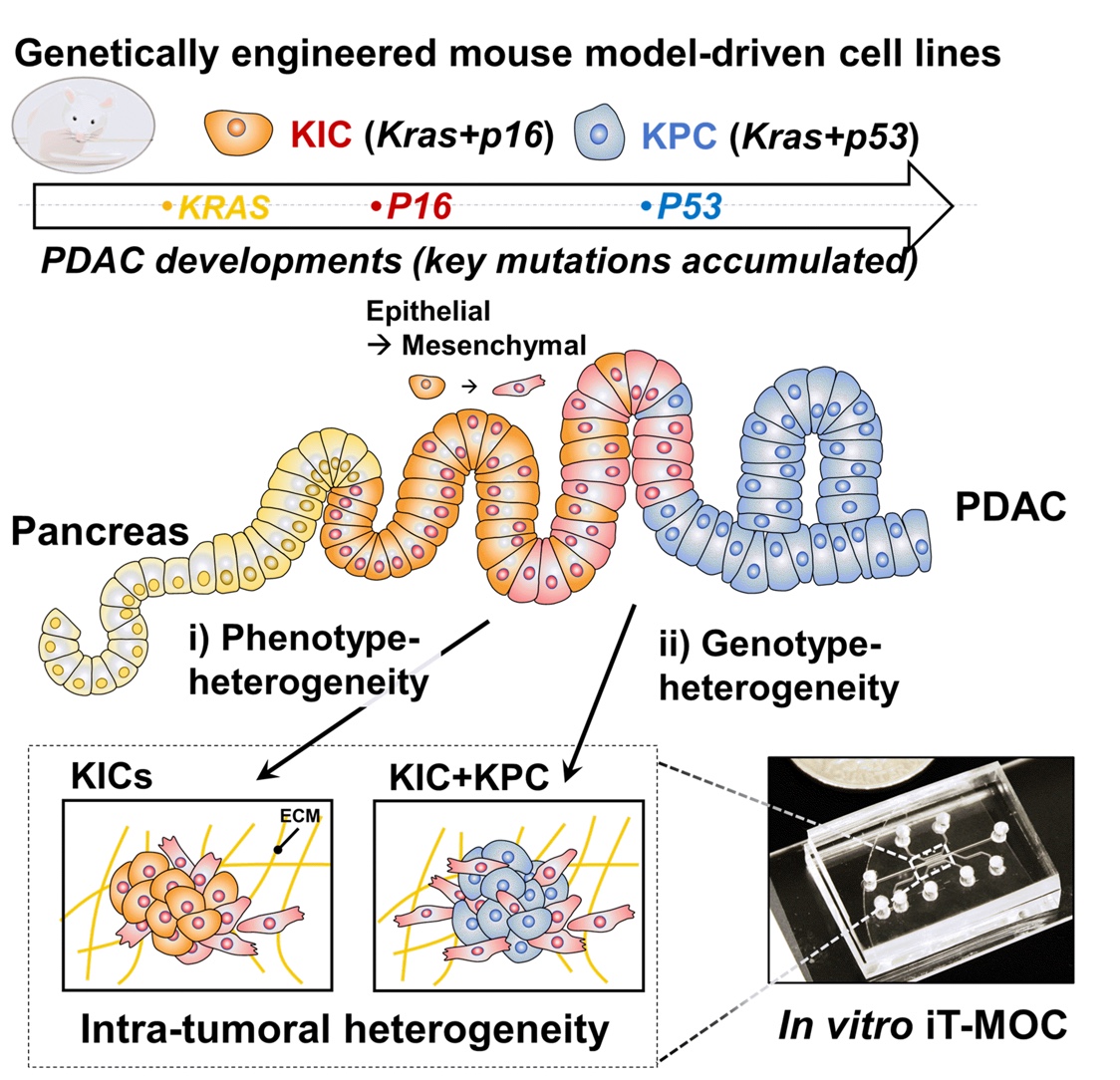
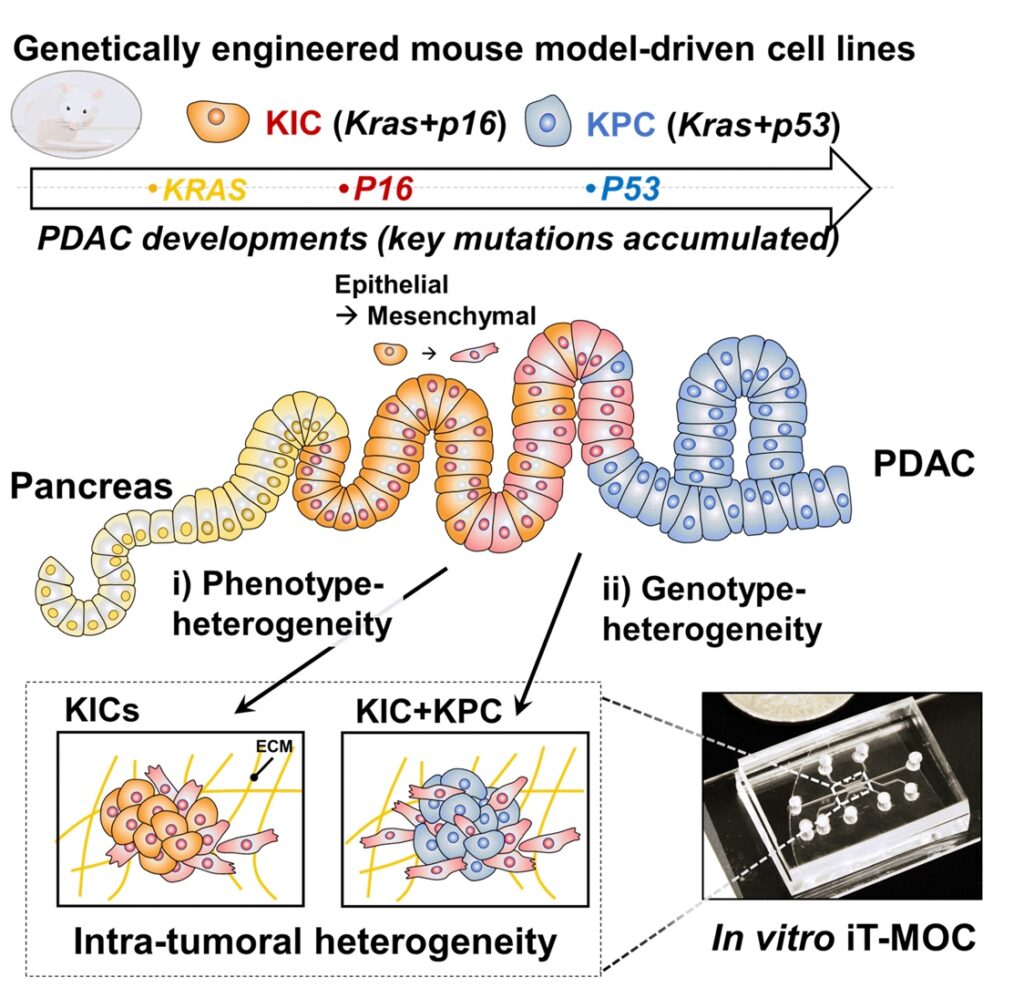
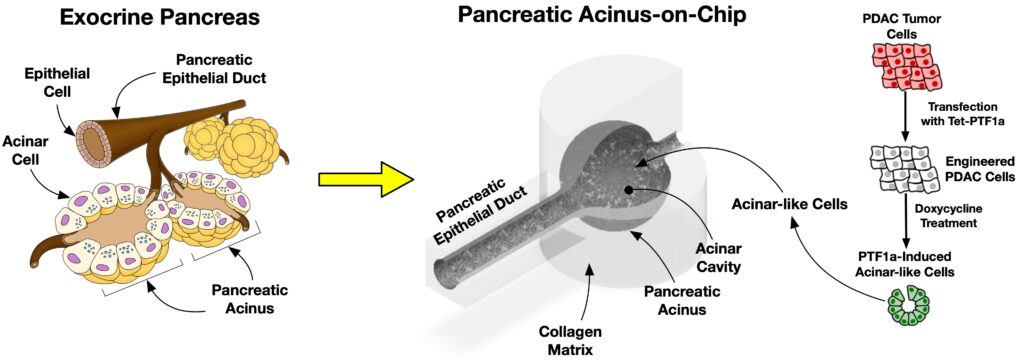
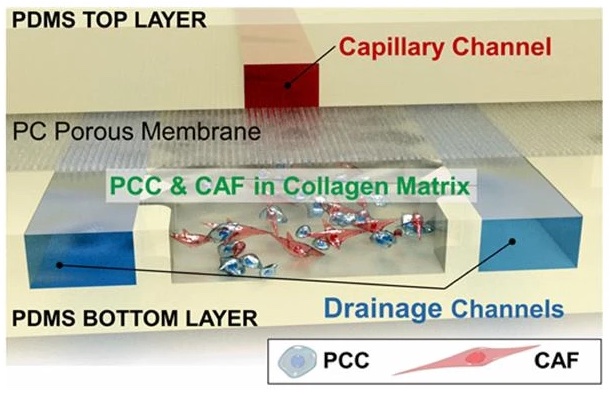
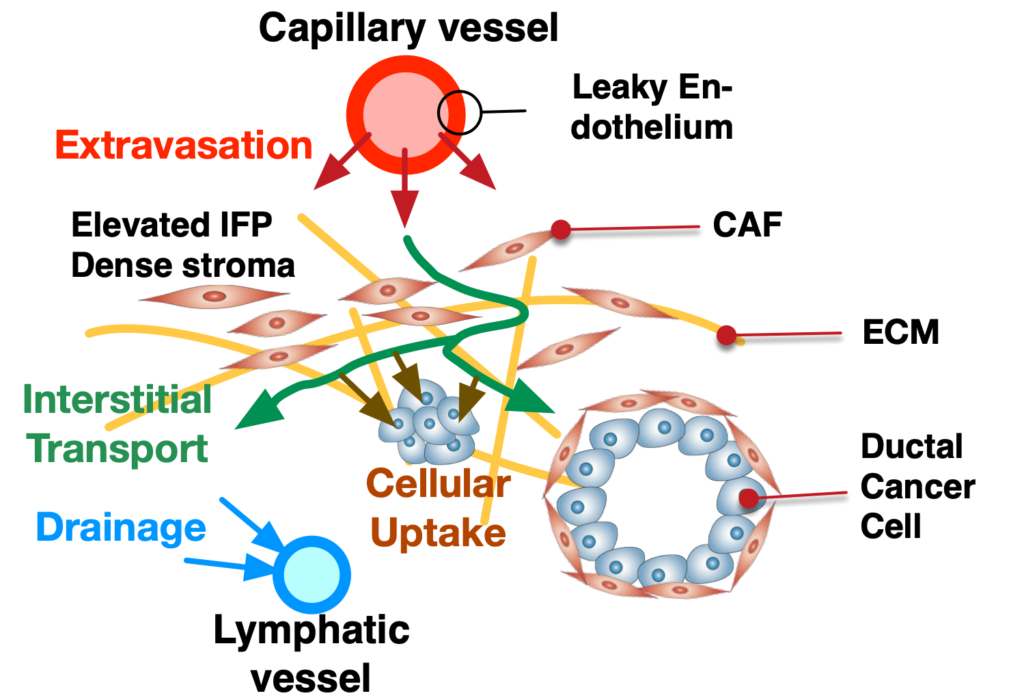
Pancreatic ductal adenocarcinoma (PDAC) is the most prevalent cancer types in pancreatic cancer. PDAC poses a significant unmet clinical need with a dismal 5-year survival rate of 13% in 2024. PDAC is highly desmoplastic tumor, of which 90% of tissue volume is stroma stissue and only 10% is occupied by cancer cells. The stroma consists of various cell types including cancer-associated fibroblasts (CAF), endothelial cells and immune cells. In addition, leaky vasculature at the PDAC TME activates extravascular coagulation/fibrinolysis systems, which are thought to promote proliferation, invasion and desmoplasia of the PDAC TME. Complex interactions among these cellular and molecular components in the stroma play two-competing roles (i.e., pro- and anti-tumor) in tumor growth, invasion and drug resistance. By developing and using microphysioloigcal systems of PDAC TME, we are discovering therapeutic strategies to reprogram the PDAC stroma by targeting the coagulation system to improve drug delivery and efficacy.
Among these cells, CAFs play multi-faceted roles in creating drug-resistant and immunosuppressive tumor microenvironment (TME). CAFs are rapidly proliferating, producing various growth factors and cytokines, and actively synthesizing extracellular matrix proteins and exerting contraction on tumor.
We are further postulating a new therapeutic strategy of targeting the coagulation system in the TME. A loss of vascular integrity and subsequent local activation of the coagulation system in the TME are consistent pathological features of many cancers, including PDAC. A leaky tumor vasculature drives the release of circulating coagulation factors into the TME. Tissue factor expressed by pancreatic cancer cells mediates the conversion of the central pro-coagulation factor prothrombin to the active serine protease thrombin, which then engages multiple downstream targets in the PDAC TME that contribute to tumor progression and formation of a robust desmoplastic stroma. My group is currently devising the microfluidic PDAC model to test the therapeutic potential of these downstream targets to reprogram the PDAC stroma