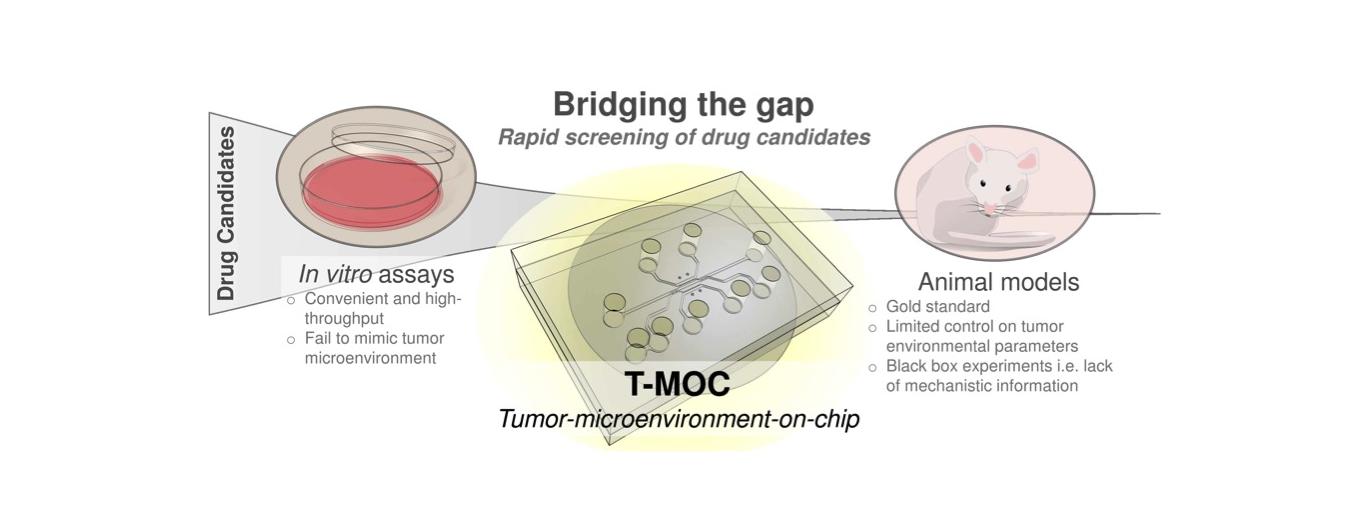
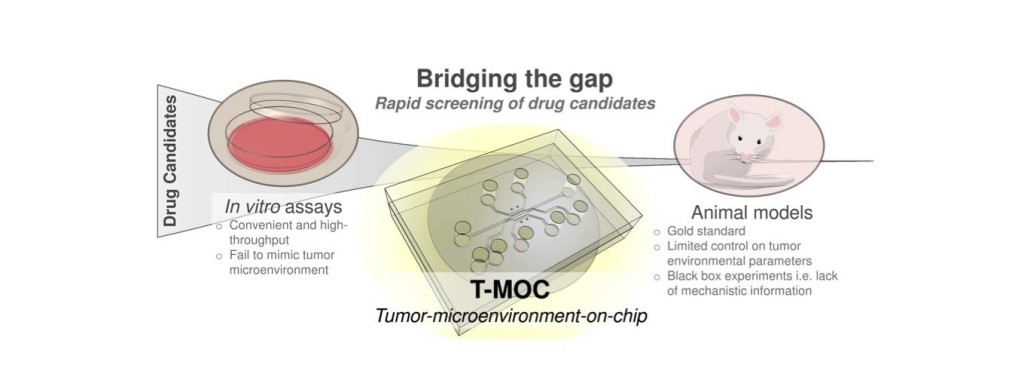
Advance of cancer treatment and personalized medicine depends on rapid and effective drug screening. In vitro assays currently used for this purpose can be convenient and high-throughput but the results are often not indicative of in vivo or clinical outcomes due to lack of culture settings specific to tumor microenvironment. Although a small animal model can provide a more realistic tumor microenvironment, it is also limited by poor control of parameters and only provides end results with limited mechanistic information. There is a need for a new model system that is capable of mimicking the in vivo tumor microenvironment with great degree of control and can also provide high-content information with potential for translation to clinical outcomes. We have been developing new in vitro tumor models using microfluidics and tissue engineering technologies, named tumor-microenvironment-on-chip (T-MOC). These models are developed to study biophysics of cancer cells and stromal tissues, and to accelerate drug screening and discovery.